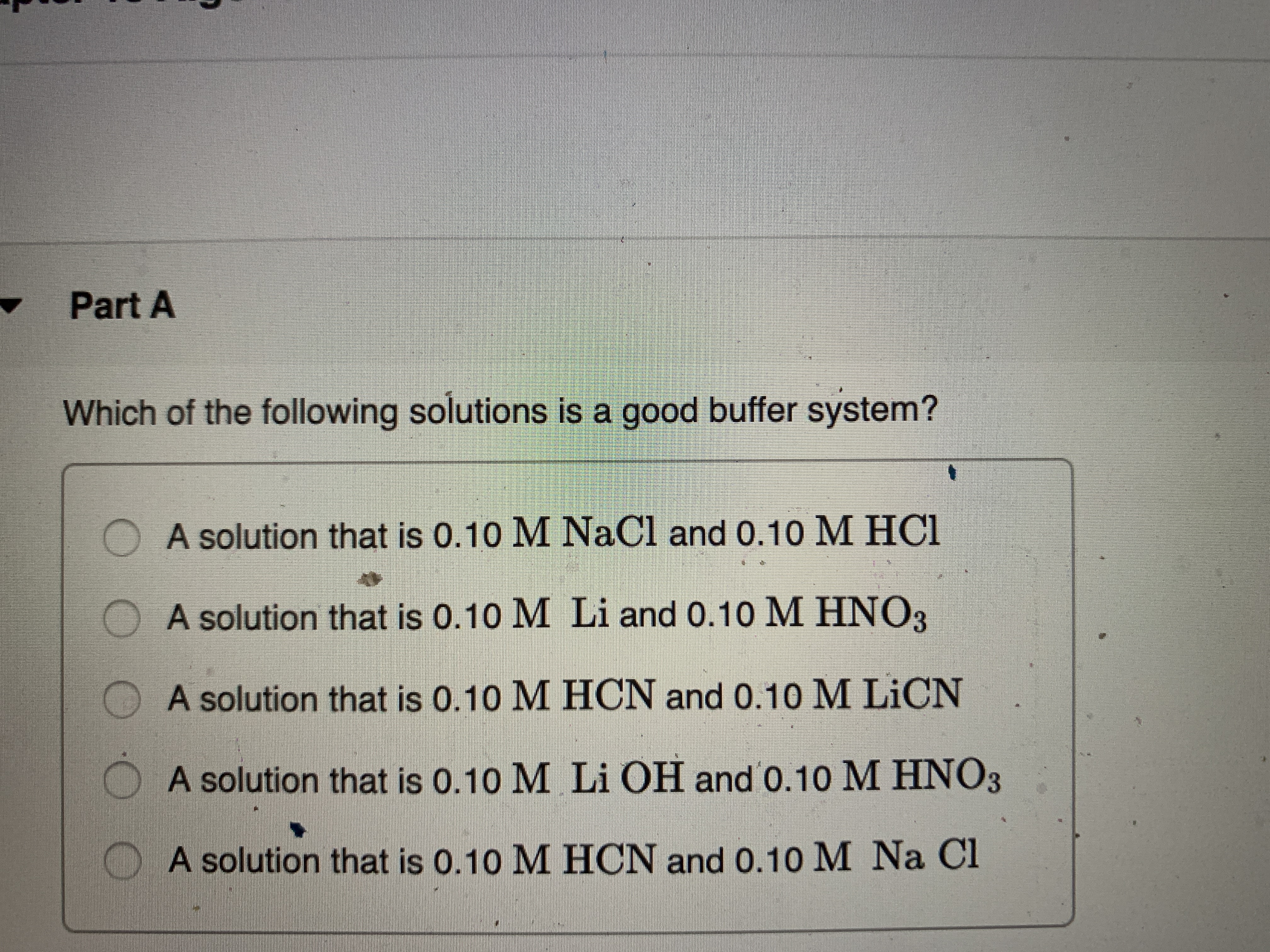
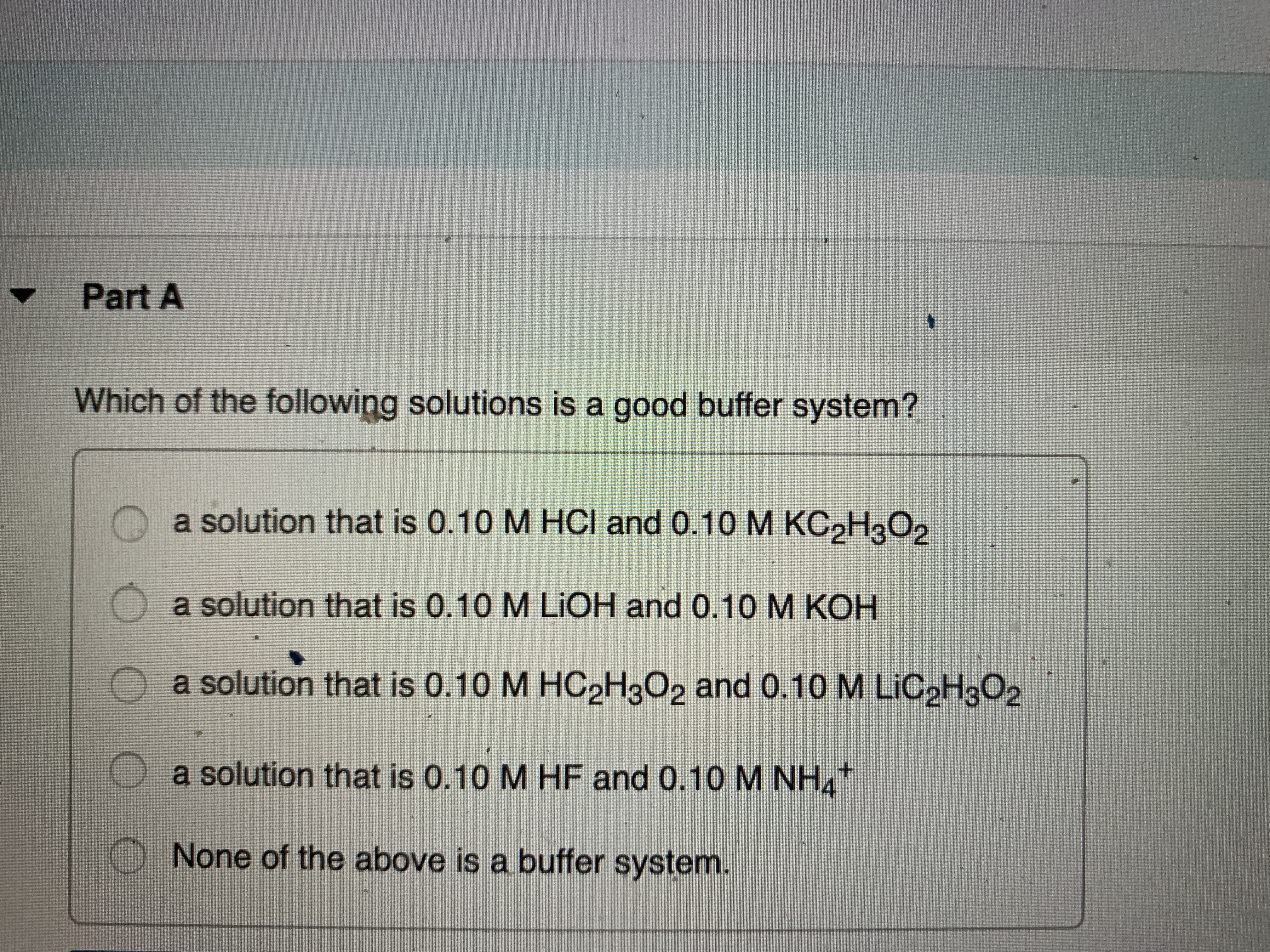
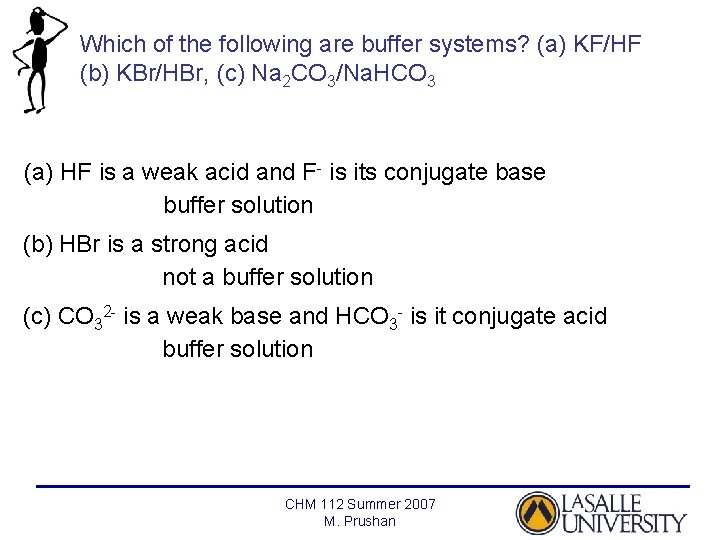
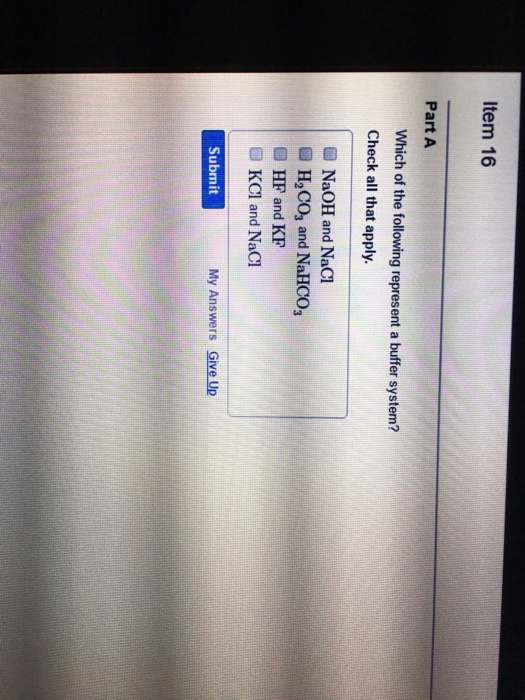
Which Of The Following Is A Buffer System?
Which of the following is a buffer system?. It can be made either in a weak acid and salt or can be a weak base and its salt. H3PO4 is a weak acid. Strong acid HCl buffered by weak base NaHCO3 One way the kidneys maintain HCO3- balance is by __________.
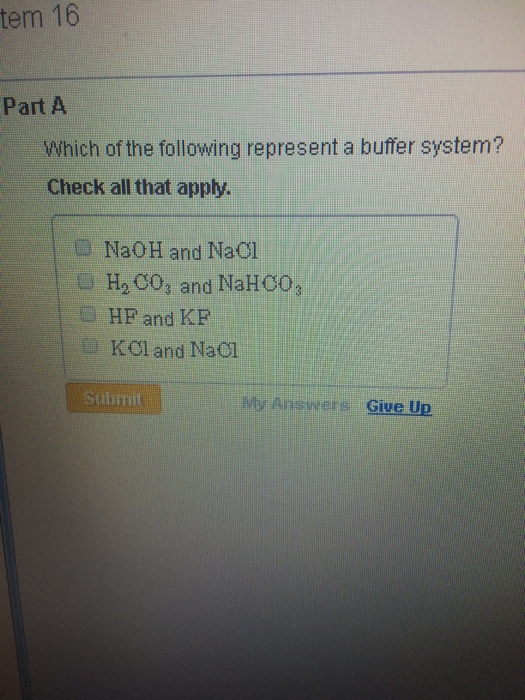
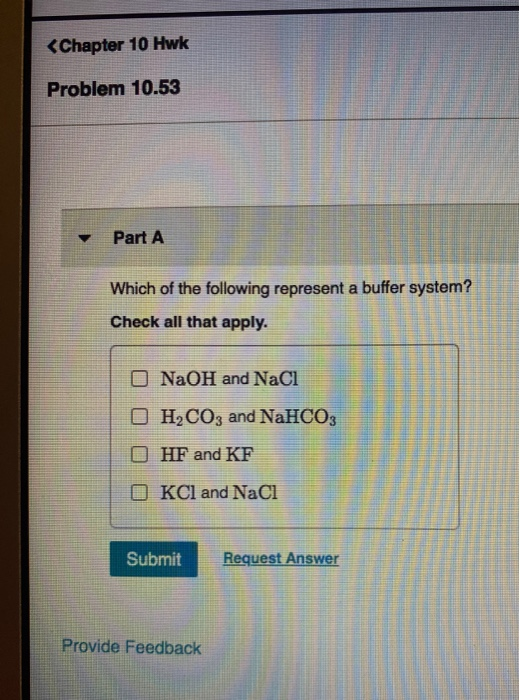
H2PO4- is the conjugate base derived from the salt. Discover what is a buffer overflow attack and how Fortinet can mitigate and prevent overflow attacks. Which of the following represent a buffer system.
It uses N-MOS technology. NaCl and NaNO 3. If acidis added to the solution it is consumed by the conjugate base.
Match the following buffer system with its appropriate example. Check all that apply. Dissolves in water only as a.
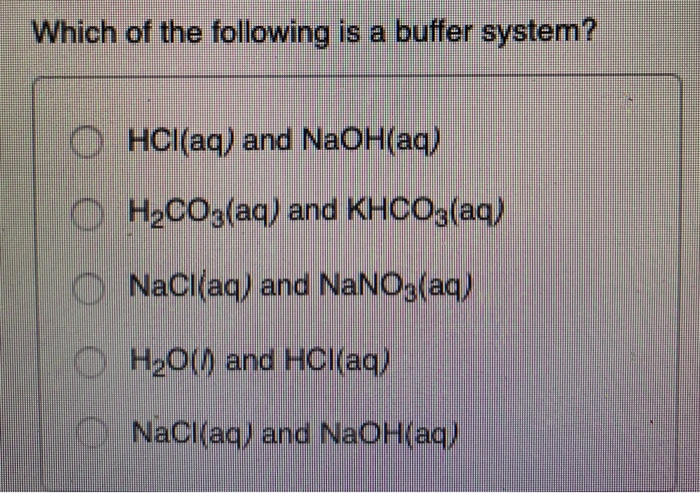
Correct Answer - B. If acidis added to the solution it is consumed by the conjugate base. A NaCl aq and NaOH aq b HCl aq and NaOH aq c H2O l and HCl aq d H2CO3 aq and KHCO3 aq e NaCl aq and NaNO3 aq Please explain answer.
Which of the following solutions is a good buffer system. Its a buffer system that consists of a weak acid and its conjugate base. It is a tri-state bi-directional 8-bit buffer which is used to interface the 825354 to the system data bus.
Loss of messages occurs between the sending process and the outgoing message buffer. Which of the following is a buffer system.
It is a tri-state bi-directional 8-bit buffer which is used to interface the 825354 to the system data bus.
A buffer consists of a weak acid and its conjugate base in roughly equal amounts. H 2 O and HCl. Data Bus Buffer. Correct Answer - B. Which of the following is a buffer system. Now when the acid is added to a buffer so the ratio is not impacted due to which it does not change sufficient for pH level. State whether each of the following solutions is a buffer system or not. Which of the following is NOT a buffer system of the body. Which of the following represent a buffer system.
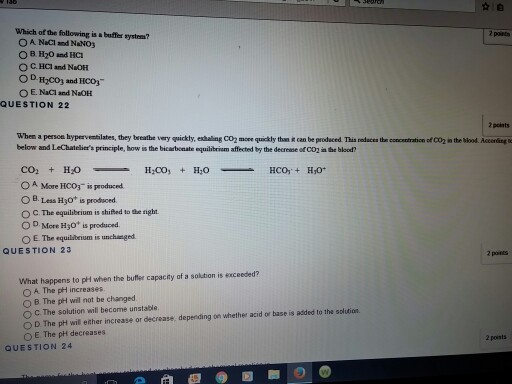
The percentage of times a page number is found in the translation lookaside buffer is called as. H3PO4 is a weak acid. A a solution that is 010 M NaOH and 010 M HNO3 B a solution that is 010 M NaCl and 010 M HCl C a solution that is 010 M HCN and 010 M NaCl D a solution that is 010 M HNO3 and 010 M. Its a buffer system that consists of a weak acid and its conjugate base. Dissolves in water only as a. In the translation look aside buffer if the page number is not present then it is called as. If acidis added to the solution it is consumed by the conjugate base.



























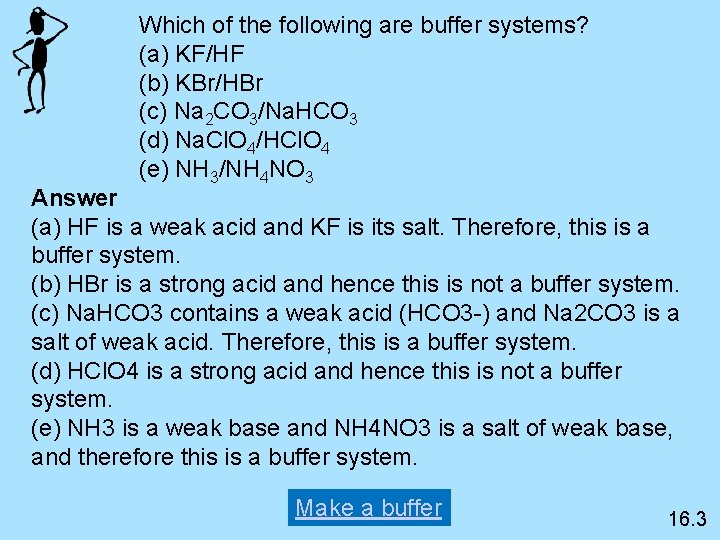


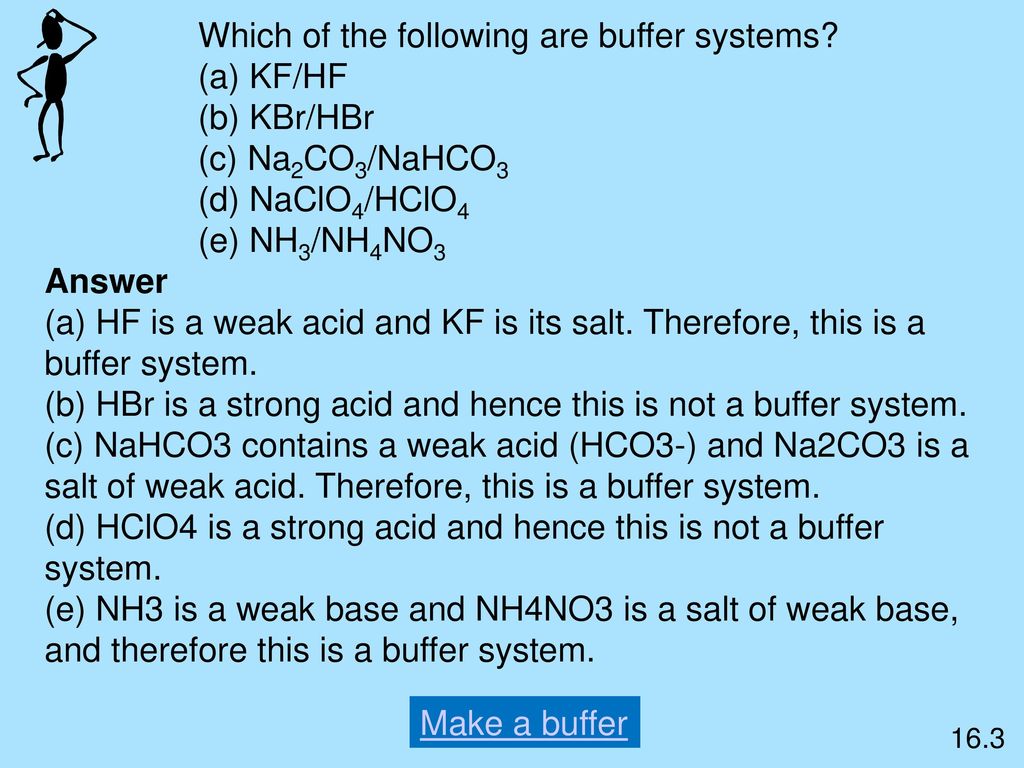









Post a Comment for "Which Of The Following Is A Buffer System?"